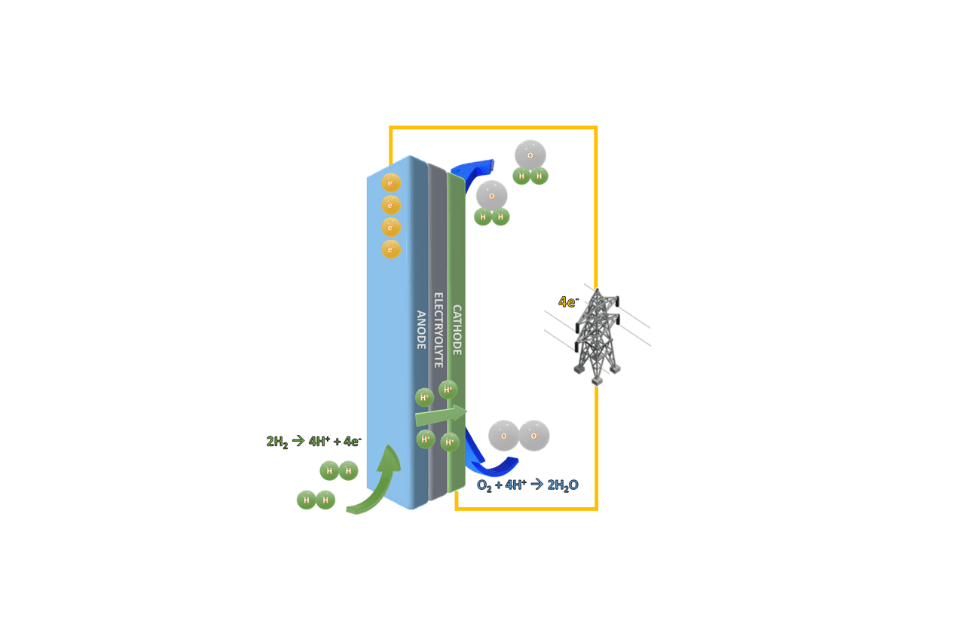
How Fuel Cells Work
ANODE
Fuel containing hydrogen is channeled to the anode where a catalyst would split the hydrogen atoms into positive hydrogen ions (protons) and negatively charged electrons.
ELECTROLYTE
The function of the electrolyte is to allow only the hydrogen ions (but not the electrons) to pass through it to the cathode. The hydrogen ions travel from the anode to the cathode through the electrolyte.
CATHODE
At the cathode, the hydrogen ions that pass through electrolyte combine with oxygen in the air to form water.
ELECTRICITY
Electrons travel along an external circuit generating an electrical current.